
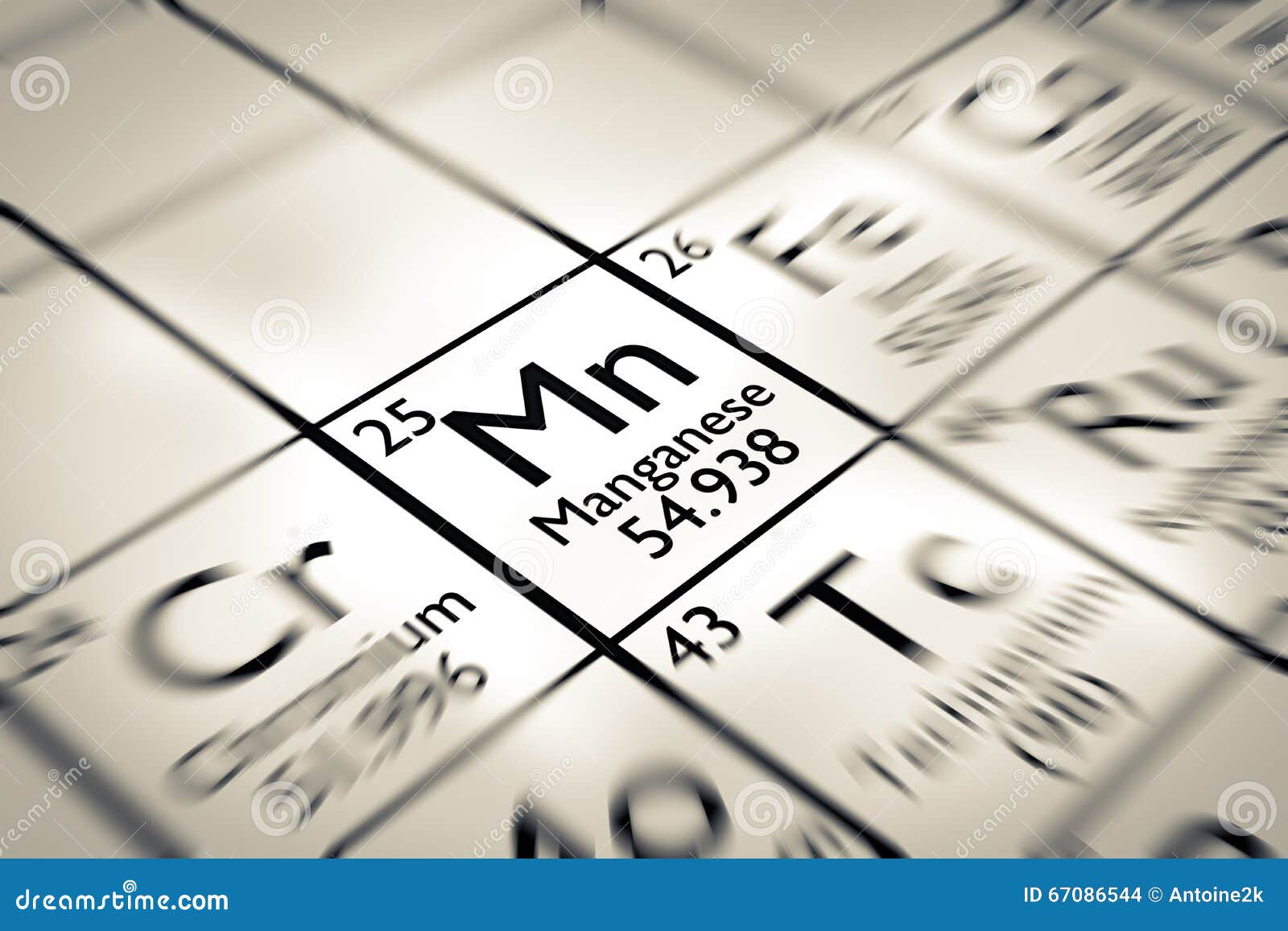
Potassium permanganate, sodium permanganate and barium permanganate are all potent oxidizers. Permanganate (+7 oxidation state) manganese compounds are purple, and can color glass an amethyst color. Manganese phosphating is used as a treatment for rust and corrosion prevention on steel. The same material also functions in newer alkaline batteries (usually battery cells), which use the same basic reaction, but a different electrolyte mixture. Manganese(IV) oxide was used in the original type of dry cell battery as an electron acceptor from zinc, and is the blackish material found when opening carbon-zinc type flashlight cells. In some preparations it is a brown pigment that can be used to make paint and is a constituent of natural umber. MnO 2 is also used in the manufacture of oxygen and chlorine, and in drying black paints. Manganese dioxide has been used since antiquity to oxidatively neutralize the greenish tinge in glass caused by trace amounts of iron contamination. Manganese(IV) oxide (manganese dioxide, MnO 2) is used as a reagent in organic chemistry for the oxidation of benzylic alcohols (i.e. The +3 oxidation state is known, in compounds such as manganese(III) acetate, but these are quite powerful oxidizing agents. The +2 oxidation state is the state use in living organisms for essential functions all of the other states are much more toxic. This oxidation state is also seen in the mineral rhodochrosite, (manganese(II) carbonate). The most stable oxidation state for manganese is +2, which has a pink to red color, and many manganese(II) compounds are known, such as manganese(II) sulfate (MnSO 4) and manganese(II) chloride (MnCl 2). The manganese in this unusual organometalic compound is in the +1 oxidation state. Methylcyclopentadienyl manganese tricarbonyl is used as an additive in unleaded gasoline to boost octane rating and reduce engine knocking. Mn 2+ often competes with Mg 2+ in biological systems, and manganese compounds where manganese is in oxidation state +7 are powerful oxidizing agents. The most common oxidation states of manganese are +2, +3, +4, +6 and +7, though oxidation states from +1 to +7 are observed. This means that, while manganese metal does not form a permanent magnet, it does exhibit strong magnetic properties in the presence of an external magnetic field. Manganese metal and its common ions are paramagnetic. It is a hard metal and is very brittle, fusible with difficulty, but easily oxidized. Manganese is a gray-white metal resembling iron. 7, 6, 5, 4, 3, 2, 1 (oxides: acidic, basic or amphoteric depending on the oxidation state)


 0 kommentar(er)
0 kommentar(er)
